The Veloci A-TEEM™ BioPharma Analyzer features innovative, column-free molecular fingerprinting technology tailored to biopharma and pharma industries. By combining chromatography's selectivity with optical spectroscopy's advantages, Veloci provides a fast, simple, and cost-effective solution for comprehensive component analysis across various industrial QC/QA life science applications.
Unlike traditional techniques such as chromatography and mass spectrometry, Veloci’s A-TEEM™ Molecular Fingerprinting technology enables component analysis without the need for time-consuming separations, simplifying workflows and significantly reducing ownership costs.
Requiring no sample preparation, this versatile tool is ideal for applications such as monoclonal antibody discrimination, cell media monitoring, vaccine characterization, protein stability analysis, and AAV quantification. Veloci enables label-free intrinsic fluorescence analysis, delivering high accuracy and efficiency in research, production, and QC/QA environments.
Key benefits
- Provides the selectivity of chromatography through advanced spectroscopic techniques
- Offers superior sensitivity compared to HPLC/MS and other spectroscopic methods such as absorbance, FTIR, and Raman
- Hassle-free operation without columns, mobile phases, or sample preparation. Eliminates solvent usage and disposal costs by removing the need for a mobile phase
- Cost-effective and user-friendly, making it suitable for both research laboratories and production environments
Applications
- Monoclonal Antibodies (mAb)
- Vaccines
- AAVs (Adeno-Associated Viruses)
- Antibody-Drug Conjugates
- Protein Stability and Aggregation
- Cell Media Monitoring and Bioreactor Monitoring
- Exosomes
EzSpec software
EzSpec provides access to recent methods and data, along with user-designated favorites for quick retrieval.
Specifications
Veloci Biopharma Analyzer. Source: HORIBA
| |
|
| Detection Limit |
Parts per billion (sample and wavelength dependent) |
| Excitation/Absorbance Wavelength Range |
200 to 800 nm |
| Fluorescence Emission Wavelength Range |
250 to 800 nm |
| Wavelength Accuracy |
+/- 1 nm |
| EEM/A-TEEM Acquisition Rate |
As fast as 60 seconds (sample and wavelength dependent) |
| Optional Accessories |
Remote fiber optic probe, Autosampler with flow cell |
| Minimum Sample Volume |
70 microliters |
| Sample Handling, Internal |
Cuvette and solid sample holders |
| Sample Handling, External |
Autosampler (96 sample maximum) |
| Light Source |
Xenon arc lamp |
| Validation |
Complies with US Pharmacopia |
| Dimensions (W x D x H) |
24 in x 17 in x 13 in; 618 mm x 435 mm x 336 mm |
| Weight |
32.72 kg (72 lbs.) |
Applications
Characterizing monoclonal antibody purification with 2D fluorescence spectroscopy
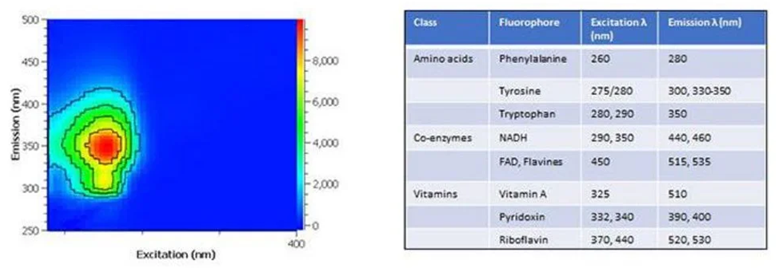
Image Credit: HORIBA
Fluorescence spectroscopy is extensively used across multiple industries, particularly in the life sciences. Two-dimensional (2D) fluorescence excitation-emission matrix (EEM) spectroscopy has been employed for over 30 years to characterize and "fingerprint" biological samples.
Monitoring cell culture media variability using the Veloci and A-TEEM molecular fingerprinting
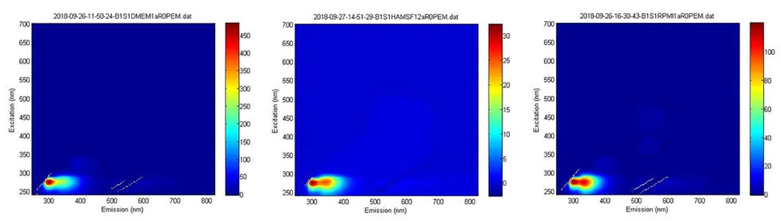 Image Credit: HORIBA
Image Credit: HORIBA
Cell culture media for bioreactors are typically prepared as aqueous solutions containing the essential nutrients and conditions necessary for optimal cell growth, product yield, and quality.
Even minor variations in media composition can significantly affect growth rates and yields. Therefore, accurate identification and analysis of cell culture media are essential for ensuring consistency and optimizing performance.
Monitoring culture medium conditions during cell proliferation using the Veloci BioPharma Analyzer
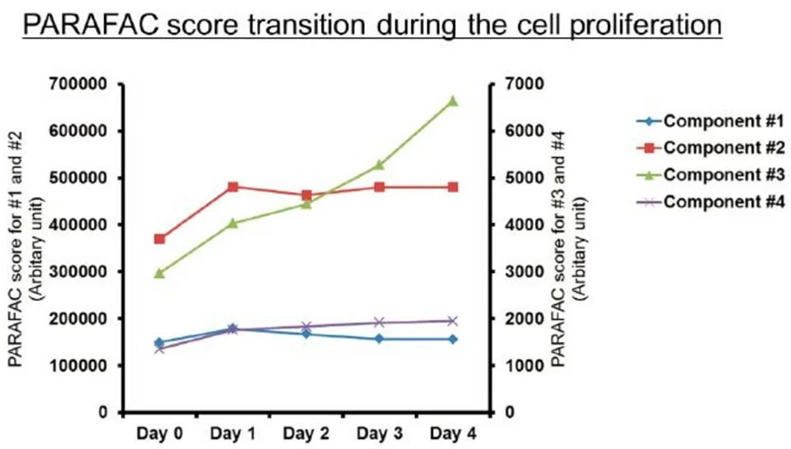
Image Credit: HORIBA
Monitoring culture media conditions is essential for optimizing cell growth in the biopharma industry. Cell health directly influences active pharmaceutical ingredients (APIs) yield and quality in applications such as regenerative medicine, monoclonal antibody (mAb) production, protein synthesis, and others.
Simultaneous A-TEEM and UV-Vis analysis for insulin structure and stability assessment
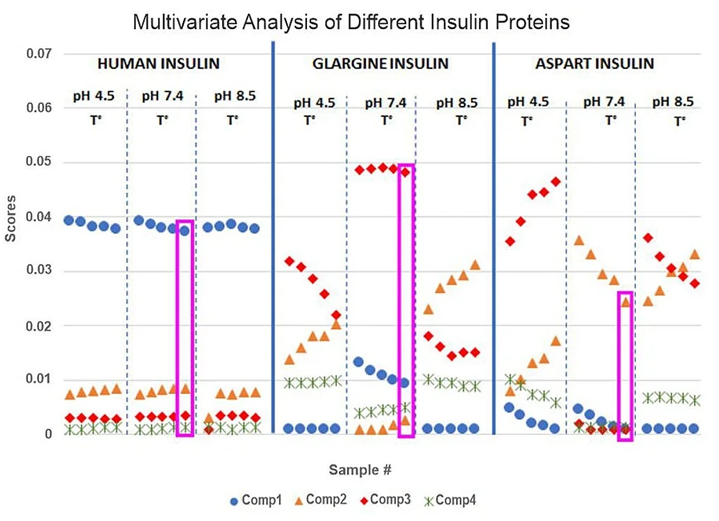
Image Credit: HORIBA
The stability and aggregation of insulin are examined using simultaneous fluorescence excitation-emission matrices (EEMs) and UV-Vis absorbance spectroscopy. Insulin, a protein hormone produced by the pancreas, is essential for regulating fundamental metabolic processes. Commercial insulin therapeutics are typically classified into two categories: short-acting and long-acting. In some cases, the distinction between these forms is defined by just one to three amino acid residues in the protein sequence.