- White paper outlines how BioDlink’s predictive digital platform reduces freeze-drying cycle development from over 60 days to under 30, enhancing scale-up precision for complex biologics
- A case study demonstrated that predicted drying times closely matched real outcomes: at −18 °C, the model projected 73.5 hours versus 75 hours experimentally, and at −15 °C, projected 64.9 hours versus an actual 66 hours
BioDlink, a leading global contract development and manufacturing organization (CDMO), has released a white paper titled Lyophilization Reimagined: A Digital‑Intelligence Platform for Predictable, Scalable Biologics Development, providing new evidence that digital intelligence tools can drastically improve the efficiency and scalability of biologics freeze-drying processes.
This white paper outlines how traditional lyophilization development, long considered a bottleneck in biologics production, is being disrupted by BioDlink’s proprietary BDKLyo™ platform. Traditionally, lyophilization process development could take over two months due to trial-and-error testing and challenges in translating laboratory results to commercial-scale consistency. The BDKLyo™ platform, based on the Pikal model, regarded as lyophilization’s 'gold standard', enables precise prediction of thermal and mass transfer conditions across scales.
Key Findings:
Development Time Halved: Digital intelligence applied via BioDlink’s proprietary BDKLyo™ platform has reduced average lyophilization cycle development time from over 60 days to fewer than 30.
Fewer Experiments, More Predictability: The number of required experimental iterations is significantly reduced, thanks to advanced modeling based on the Pikal equation, which accurately predicts critical parameters such as chamber pressure, shelf temperature, and primary drying duration.
Increased Scale-Up Success Rates: The digital approach enables consistent product quality across development stages and scales, lowering the risk of revalidation and manufacturing setbacks.
This shift is particularly significant as the biologics market grows increasingly complex, with monoclonal antibodies, antibody-drug conjugates, and recombinant proteins dominating new product pipelines. Scale-up precision and manufacturing reproducibility have become critical, especially for molecules with narrow formulation margins. The digital modeling approach described in the paper enables developers to simulate process outcomes in silico, minimizing costly experimental runs and increasing first-time-right outcomes in manufacturing.
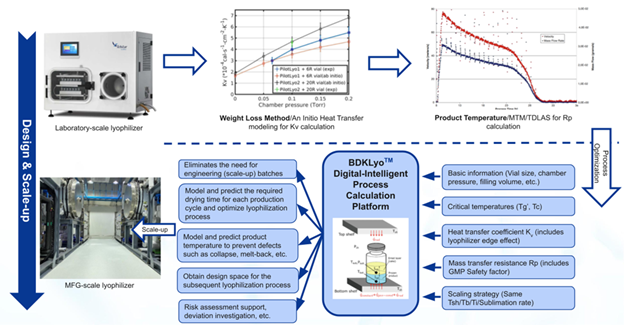
According to this white paper, the integration of predictive modeling not only shortens development timelines but also significantly reduces risk during technology transfer. Early identification of critical parameters such as shelf temperature, chamber pressure, and product resistance allows manufacturers to anticipate and control key quality attributes, improving batch-to-batch consistency. Case studies' key findings include, based on real experimental validation data:
In Case Study A, adjusting the shelf temperature from −25 °C to −20 °C based on digital predictions shortened primary drying time from 56 hours to 39 hours, with actual results of 36.4 hours within 10 percent of model forecasts and product temperatures remaining consistent.
In Case Study B, optimization of large‑volume fills demonstrated that predicted drying times closely matched real outcomes: at −18 °C, the model projected 73.5 hours versus 75 hours experimentally, and at −15 °C, projected 64.9 hours versus an actual 66 hours.
These case studies demonstrate the ability of the BDKLyoTM digital-intelligence process calculation platform to accelerate lyophilization process development and scale-up. With BDKLyoTM, IND-stage projects can be completed in 1-2 experimental rounds, saving time and money during the CMC phase.
Download the full white paper here: https://bit.ly/4qez3si